Here is a sneak peek of how to ‘Interpret Ionisation Energy Graphs’, a method that students will learn during their H2 Chemistry tuition class with Ms Sim.
First Ionisation Energy definition
The first ionisation energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms to produce 1 mole of gaseous singly positive charged ions.
Na(g) → Na+(g) + e 1st I.E.= +494 kJ mol-1
Note that the state symbols must be written and it should be in gaseous state.
The 1st I.E. Graph
The 1st ionisation energy trend of different elements across a period is often tested in the atomic structure topic. Students are required to know how to sketch the graph and to provide explanations for the trend. This is actually a bookwork question.
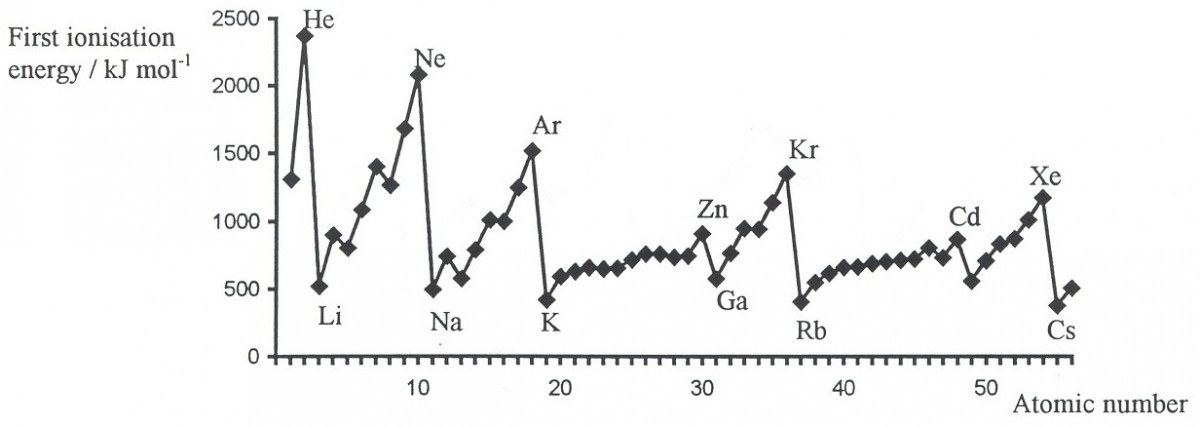
The 2nd/ 3rd/ 4th I.E. Graphs
To test students at a higher level, questions related to interpreting a 2nd or even 3rd ionisation energy graphs may be given. Some students will attempt by trying to shift the graphs or shift the elements on the graph but it can be slightly confusing if they can’t remember which direction to shift and besides that, they may face problems if asked to explain the graph.
In Ms Sim’s classes, students will learn a method to handle such questions and with that, students will have no problems interpreting a 2nd, 3rd, 4th I.E. graphs etc. They will be able to explain the graph with ease using what they have learnt for 1st I.E. graph as the explanation is actually not something new.
Click on the button below to enrol.
Please check out our other tutor who specializes in H2 Physics tuition in Singapore and H2 Maths tuition in Singapore.

